ASCO CARBON DIOXIDE INC | 5409 Highway Ave. | Jacksonville, FL 32254 | T +1 904 278-6503 |
ascoco2.comVersion 3.3 (02/18)
6
From Liquid CO
2
into Dry Ice
General Information about CO
2
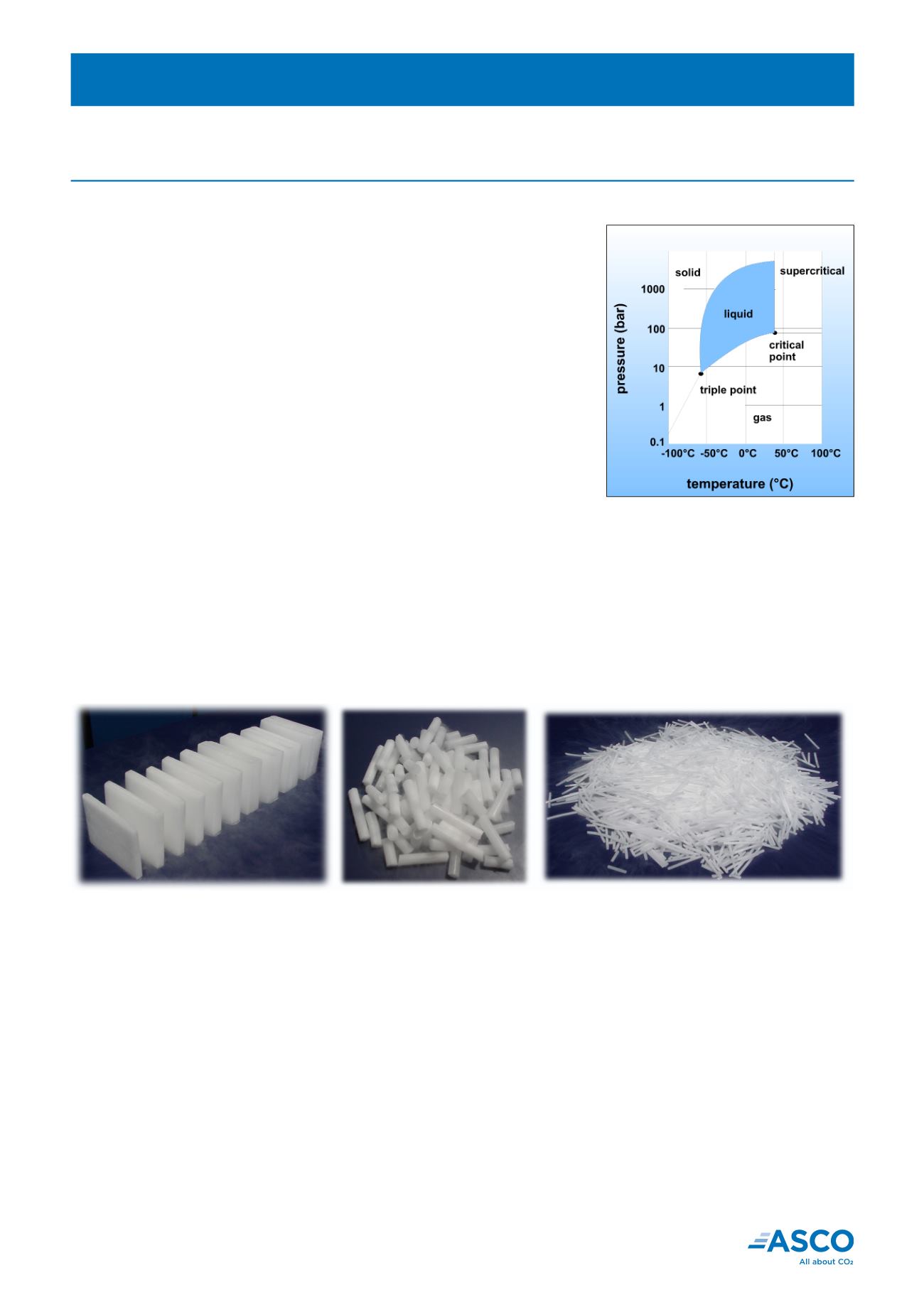
Carbon Dioxide has 3 physical states of gas, liquid and solid which depend
on temperature and pressure.
The relationshipbetween the3states is shownon thepressure-temperature
phase diagram beside.
Where does CO
2
come from?
CO
2
is derived from a number of sources including combustion of carbo-
naceous fuels, fermentation, natural wells, and as a by-product of industrial
processes such as ethylene oxide and bioethanol production and ammonia
synthesis.
CO
2
phase diagram
The Solid State (Dry Ice)
Below the triple point (5.18bar, - 56.6 °C) (75.13psi) CO
2
can only appear in its solid and gaseous state. Dry Ice is
the common trade name for solid CO
2
. At atmospheric pressure it has a temperature of approx. -79 °C. The solid
CO
2
changes directly into its gaseous state. This evaporation (sublimation) does not leave any residues. Dry ice is
non-toxic, non-inflammable, inert, without smell and bacteriostatic. It is white and has a density of approx. 1'500kg/m
3
(93.6 lb/ft
3
) in its compact state. Dry ice is an ideal refrigerant which qualifies especially well for various applications.
It has a high cooling capacity and heat transfer is very high when in direct contact with the cold material.
Dry ice slices and blocks
16mm (5/8 in) pellets
3mm (1/8 in) pellets
ASCO
has the best range of dry ice machines for dry ice production.
The Liquid State
Within a temperature range between -56.6 °C and 31 °C and pressure greater than 5.2bar (75.42psi) and less than
74bar (1'073.28psi) respectively CO
2
is in its liquid state except at very high pressures. This means that, below
5.2bar (75.42psi), CO
2
exists only in its solid or gaseous state. At 5.2bar (75.42psi) and - 56.6 °C all thee states
(solid, liquid and gas) are present. This is called the triple point.